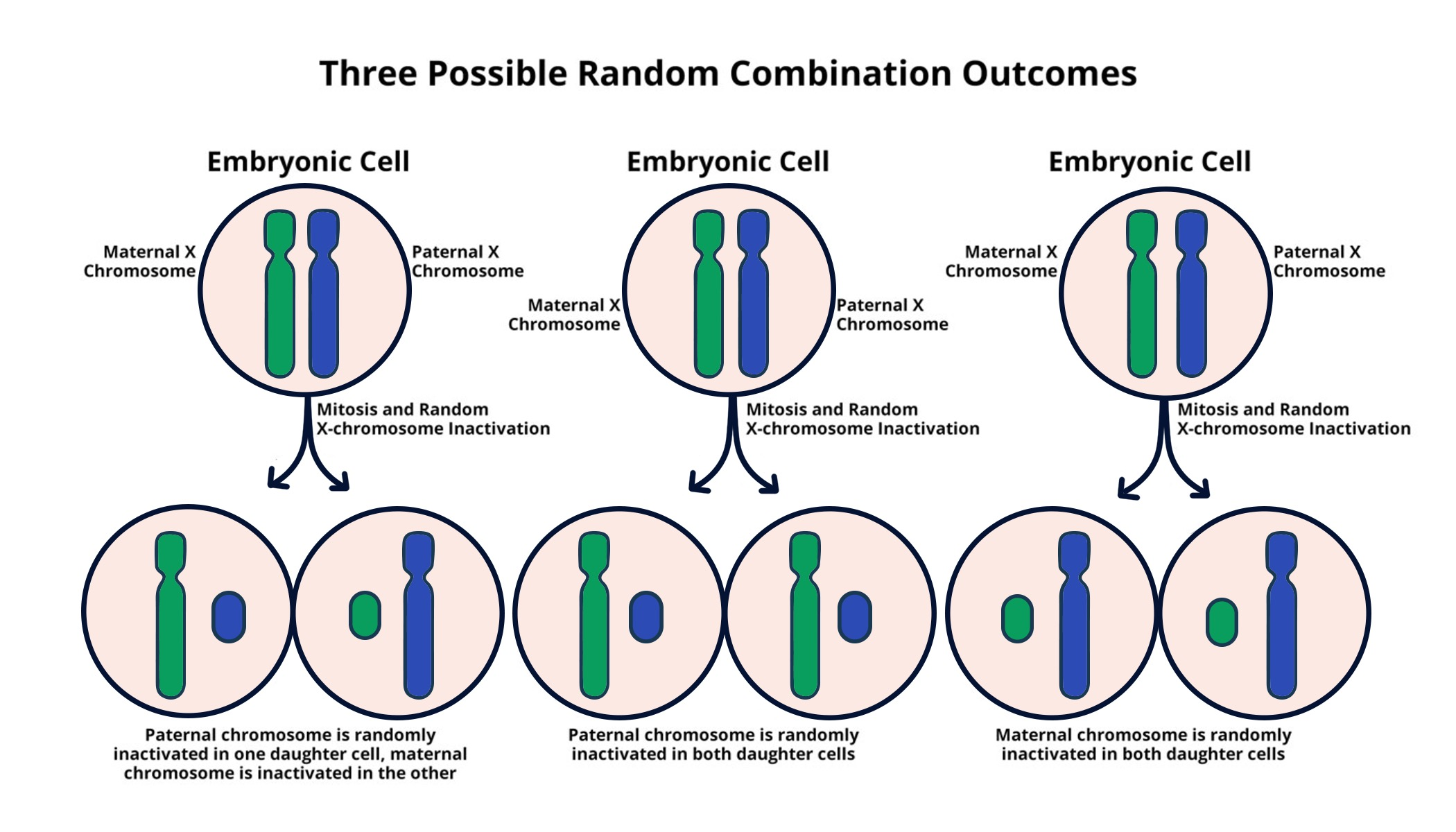
X chromosome inactivation is a fascinating biological process that plays a critical role in human genetics. This mechanism ensures that females, who possess two X chromosomes, effectively silence one of them to prevent an overproduction of genes. The significance of X chromosome inactivation extends to various genetic disorders, including Fragile X Syndrome and Rett Syndrome, both of which arise from mutations on the X chromosome. By understanding how chromosomal silencing occurs, researchers seek to unlock new treatments aimed at these conditions. The incredible work taking place in labs, such as those led by Jeannie Lee, is paving the way for potential therapies that utilize the mechanisms behind X-linked genes to alleviate symptoms or even cure these debilitating disorders.
Often referred to as the process of lyonization, X chromosome inactivation is essential for balancing gene expression between genders. While males are equipped with a single X chromosome, females utilize this regulatory mechanism to adjust the dosage of X-linked genes. This intricate process not only underlies the normal functioning of cells but also has profound implications for various chromosomal disorders, such as Fragile X Syndrome and Rett Syndrome. Advancements in understanding how genes become dormant on the X chromosome can lead to significant breakthroughs in treating these genetic conditions. As ongoing research uncovers the complexities of chromosomal silencing, the prospect of developing effective therapies becomes increasingly promising.
Understanding X Chromosome Inactivation
X chromosome inactivation (XCI) is a crucial biological process that allows female mammals to maintain gene dosage parity with males. In essence, XCI randomly inactivates one of the two X chromosomes in each female cell, ensuring that males and females express similar levels of X-linked genes. This phenomenon is not just a hallmark of gender difference; it plays a significant role in regulating certain genetic disorders. Understanding the intricate mechanisms behind XCI offers vital insights into potential therapies for conditions like Fragile X Syndrome and Rett Syndrome.
Researchers, led by notable scientists like Jeannie Lee, have made significant strides in unraveling the complexities of X chromosome inactivation. This intricate process involves chromosomal silencing, where one X chromosome is coated with a unique RNA molecule known as Xist, which alters the biophysical properties of the chromosomal environment. This alteration triggers a cascade of interactions that ultimately lead to the silencing of the inactive X chromosome, a mechanism crucial for preventing the overexpression of genes and mitigating potential harmful effects.
Frequently Asked Questions
What is X chromosome inactivation and how does it affect genetic disorders like Fragile X Syndrome?
X chromosome inactivation is a biological process where one of the two X chromosomes in female cells is silenced to prevent overexpression of X-linked genes. This mechanism is crucial for proper gene dosage and is relevant in genetic disorders like Fragile X Syndrome, where mutations on the X chromosome can lead to intellectual disabilities. By understanding X chromosome inactivation, researchers aim to develop therapies that can reactivate silenced genes and provide relief for affected individuals.
How do researchers study X chromosome inactivation in relation to Rett Syndrome?
Researchers, including those in Jeannie T. Lee’s lab, study X chromosome inactivation by exploring the mechanisms involved in chromosomal silencing. They focus on the role of RNA molecules like Xist, which change the physical properties of the chromosomal environment, making it easier for the inactivation process to occur. Insights into X chromosome inactivation not only deepen our understanding of Rett Syndrome but also pave the way for potential treatments that target X-linked genes to restore their functionality.
What role does the Jell-O-like substance play in X chromosome inactivation?
The Jell-O-like substance mentioned in studies of X chromosome inactivation refers to a gelatinous coating that surrounds chromosomes, creating an environment necessary for chromosomal silencing. During X chromosome inactivation, an RNA called Xist interacts with this substance to alter its properties, facilitating the inactivation process. This mechanism is vital for understanding how cells regulate X-linked genes and offers potential therapeutic routes for genetic disorders such as Fragile X and Rett syndromes.
Can treatments for X-linked genetic disorders restore function to silenced X chromosomes?
Yes, recent approaches developed in the context of X chromosome inactivation research aim to unsilence inactivated X chromosomes, potentially restoring the function of healthy genes that had been silenced. This is particularly significant for individuals with X-linked genetic disorders like Fragile X Syndrome and Rett Syndrome, where only one X chromosome carries a mutation. By targeting the inactivation process, therapies could re-enable the expression of healthy genes, thereby alleviating symptoms of these conditions.
How does X chromosome inactivation impact gene therapy for diseases linked to X-linked genes?
X chromosome inactivation presents unique challenges and opportunities for gene therapy targeting X-linked genes. By silencing one X chromosome in females, it effectively limits the gene expression to that present on the active X chromosome. Understanding this process allows scientists to devise strategies to reactivate silenced genes, which is advantageous for developing gene therapies for conditions like Fragile X Syndrome and Rett Syndrome, where correcting one mutated gene can make a significant difference in patient outcomes.
| Key Aspects | Details |
|---|---|
| X chromosome uniqueness | Females have two X chromosomes, while males have one, creating a need for one X chromosome inactivation in females. |
| Role of Xist | Xist RNA molecule initiates the inactivation process of one X chromosome by altering the surrounding chromatin structure. |
| Jell-O-like substance | The ‘Jell-O’ around chromosomes allows separation and prevents tangling, crucial for the X-inactivation process. |
| Therapeutic potential | The research may lead to treatments for disorders like Fragile X Syndrome and Rett Syndrome by unsilencing mutated X chromosome genes. |
| Cellular implications | Freeing inactivated X chromosomes could restore function to genes affected by mutations, suggesting minimal side effects. |
| Future research aims | Further optimization and safety studies are planned before moving into clinical trials. |
Summary
X chromosome inactivation is a critical biological process that allows females to use only one of their two X chromosomes, effectively silencing the other. Recent research led by Jeannie T. Lee has deepened our understanding of how this inactivation occurs, revealing the pivotal role of the Xist RNA gene and a unique gelatinous substance surrounding chromosomes. This groundbreaking work not only elucidates fundamental questions in cell biology but also opens new avenues for treating genetic disorders like Fragile X and Rett syndromes. The potential to restore function to mutated genes without affecting healthy genes suggests a promising future for therapeutic interventions.