X chromosome inactivation is a fascinating biological process by which females silence one of their two X chromosomes to balance gene dosage with males who have only one X. This process not only has implications for sex determination but is also pivotal in understanding various genetic diseases associated with the X chromosome, such as Fragile X Syndrome and Rett Syndrome. Recent research has shed light on the molecular mechanisms underpinning X inactivation, particularly the role of Xist RNA, a key player that orchestrates the silencing process. The discoveries surrounding X chromosome inactivation open up exciting avenues for gene therapy, potentially allowing scientists to unsilence certain genes that could treat or alleviate symptoms of these genetic disorders. As research continues, the hope is to translate these findings into effective treatments that could change the lives of many affected individuals.
The silencing of one X chromosome in females, known as X chromosome inactivation, represents a crucial regulatory mechanism distinct from typical gene expression controls. It poses unique challenges in genetic studies and therapy development, particularly in conditions like Fragile X and Rett syndromes, which are linked to abnormalities on the X chromosome. In this context, researchers are increasingly focusing on innovative strategies to manipulate X inactivation and its associated processes, including the pivotal role of Xist RNA in gene regulation. The potential for breakthroughs in gene therapy using insights gained from studying X inactivation holds promise for many individuals with genetic diseases. This research trajectory not only aims to decode complex biological interactions but also aspires to deliver tangible therapeutic advancements.
Understanding X Chromosome Inactivation
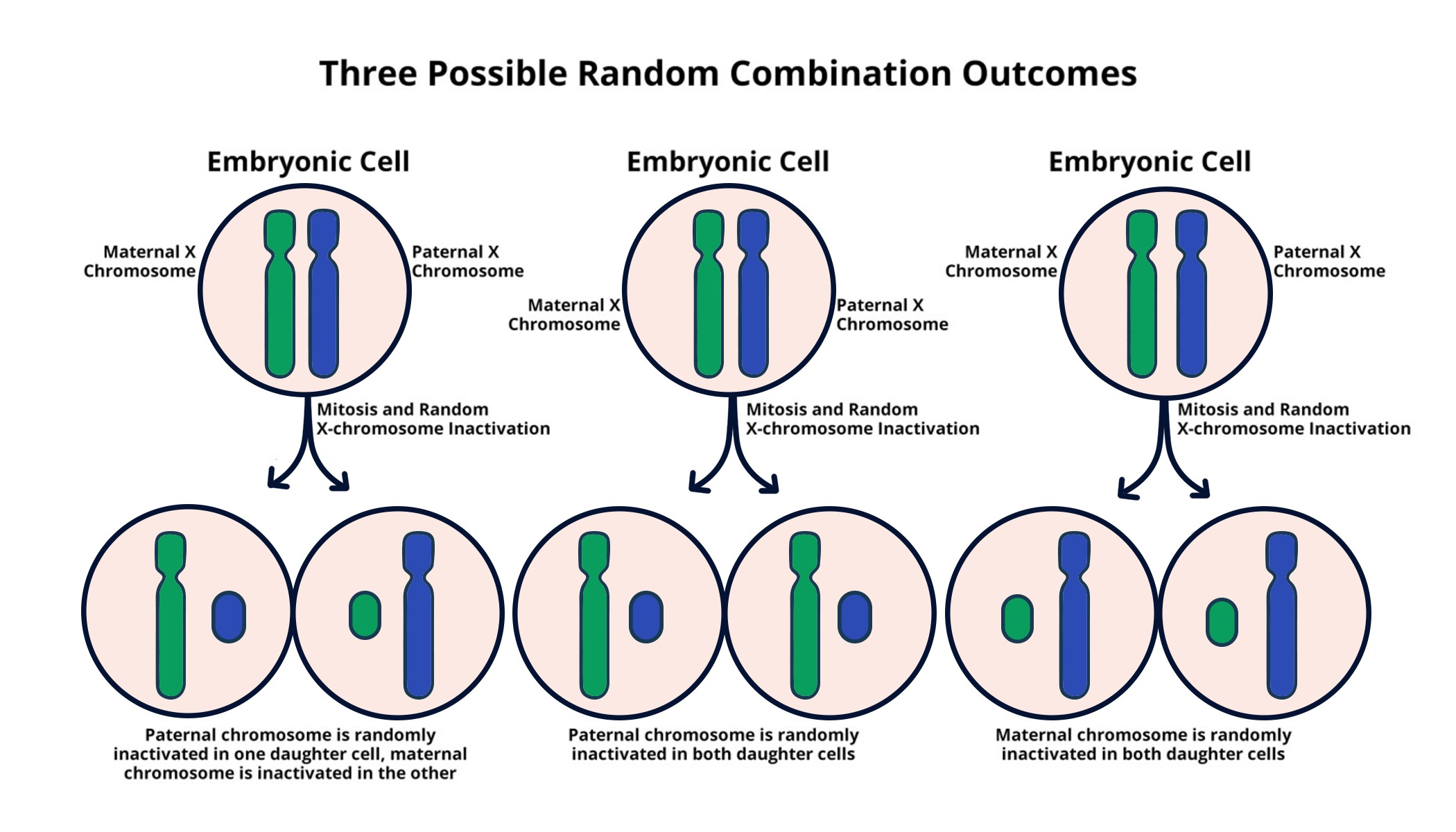
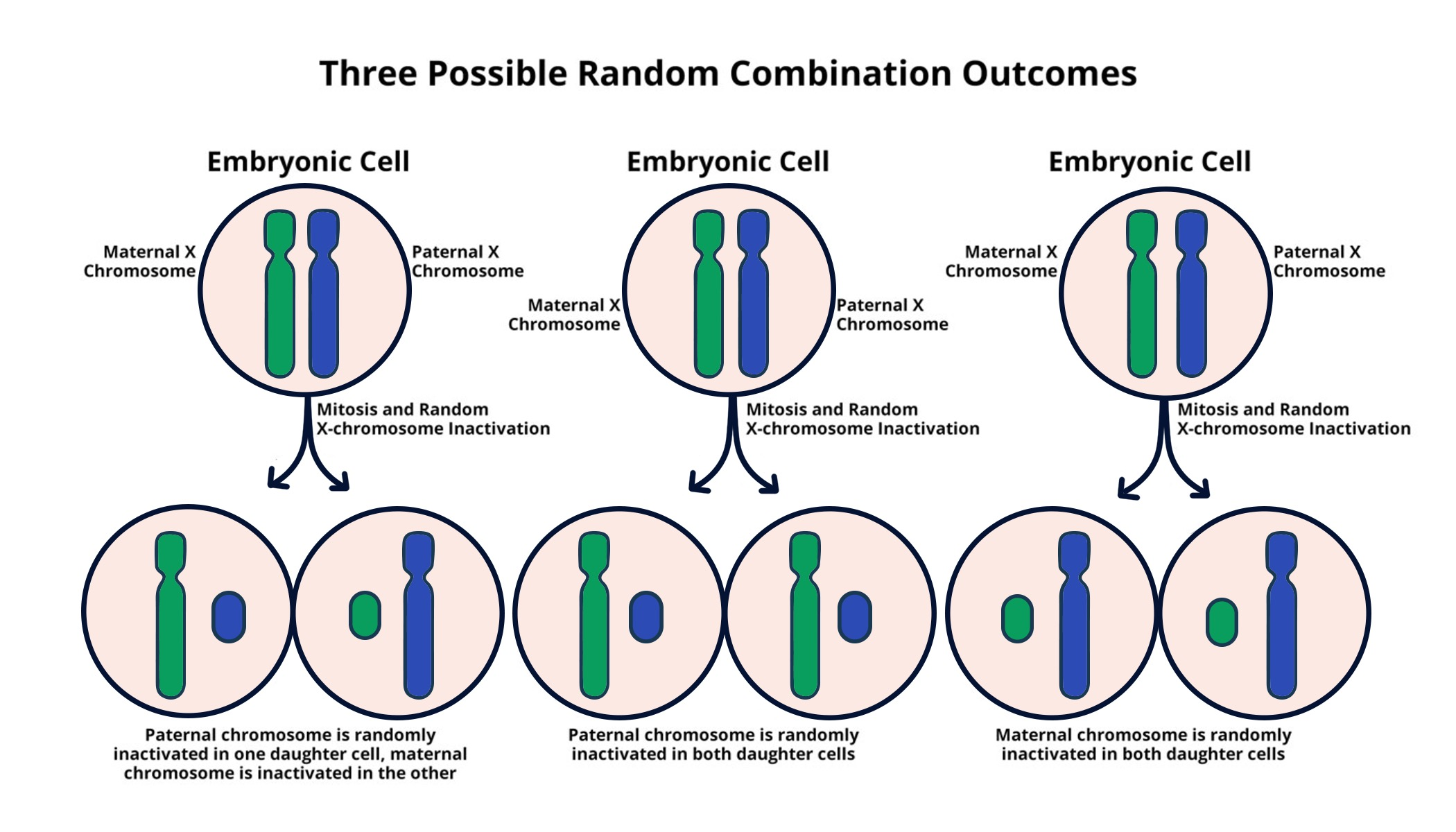
X chromosome inactivation is a critical process that occurs in female mammals, allowing for the regulation of gene expression. Each female cell randomly inactivates one of its two X chromosomes to ensure a balanced dosage of X-linked genes compared to male cells, which possess only one X chromosome. This intricate mechanism is crucial for maintaining normal development and function, as it prevents an overexpression of genes that could lead to potential health issues.
Research led by Jeannie Lee has shed light on the role of Xist RNA in this inactivation process. Xist is a long non-coding RNA that coats the X chromosome destined for inactivation, modifying the surrounding chromatin structure and rendering it transcriptionally silent. Understanding the nuances of X chromosome inactivation not only provides insight into basic biological processes but also opens the door to therapeutic avenues for genetic disorders linked to X chromosome mutations.
Implications for Genetic Diseases
Genetic disorders linked to the X chromosome, such as Fragile X Syndrome and Rett Syndrome, underscore the importance of effective gene regulation mechanisms. Fragile X Syndrome is characterized by a mutation in the FMR1 gene, leading to intellectual disability, while Rett Syndrome, often caused by mutations in the MECP2 gene, impairs normal neurological development. The inactivation process, while essential for normal female physiology, can complicate the expression of these mutated genes, effectively silencing the healthy copies that the body needs to function properly.
The breakthroughs in understanding how X chromosome inactivation works could pave the way for innovative gene therapies aimed at unsilencing these critical genes. By leveraging insights from Jeannie Lee’s research on Xist RNA and its role in modifying chromosomal environment, researchers hope to develop targeted treatments that could alleviate symptoms and restore functionality for those affected by these debilitating conditions.
The Role of Xist RNA in Gene Therapy
The discovery of Xist RNA’s function in X chromosome inactivation has significant implications for the development of gene therapies aimed at treating genetic diseases. By exploiting the cellular mechanisms that lead to chromosomal silencing, scientists can create strategies to restore expression of essential genes. For instance, in the context of Fragile X Syndrome, therapeutic interventions that effectively harness Xist’s properties may allow the healthy version of the gene to become active, providing a much-needed remedy for individuals with this condition.
Moreover, the ability to manipulate the inactivation process could also mean a more effective approach to treating Rett Syndrome. As these diseases predominantly affect females, targeting Xist RNA to control gene expression could result in more effective treatments with reduced side effects by ensuring that only dysfunctional genes are silenced, thereby preserving the function of healthy genes. This targeted approach marks a promising frontier in gene therapy development, potentially reshaping the treatment landscape for genetic disorders.
Potential Clinical Trials and Future Directions
The progression from fundamental research to potential clinical applications marks an exciting chapter in the fight against X-linked genetic disorders. As Jeannie Lee and her team make strides towards optimizing their methodologies for X chromosome manipulation, the goal to transition their findings into clinical trials becomes increasingly feasible. This progression suggests a hopeful horizon for individuals affected by Fragile X and Rett Syndromes, as potential therapeutic options could soon become available.
As the research advances, careful consideration will be necessary to assess the safety and efficacy of these emerging treatments. Initial studies focusing on animal models will provide crucial insights into how well these therapies function within a biological system. Furthermore, collaboration with regulatory agencies and patient communities will be essential in ensuring that these therapies meet the needs of those impacted by genetic diseases, ultimately paving the way for groundbreaking advancements in genetic medicine.
Exploring Chromosomal Mechanisms in Depth
Understanding the intricate chromosomal mechanisms that govern gene expression is crucial for comprehending how genetic diseases manifest. The role of chromatin structure in cellular function cannot be understated; for instance, the gooey consistency of the ‘chromosomal Jell-O’ described in Lee’s research plays a vital role in maintaining chromosomal integrity. This gelatinous material aids in organizing chromosome territories and allows cellular components to interact more efficiently, thus preserving proper gene regulation.
Investigating how chromosomal environments influence disease states could lead to targeted interventions that modify these environments. For example, the ability to alter the biophysical properties of the surrounding chromatin could enhance or reduce the expression of genes linked to various disorders. By focussing attention on structural genomics and chromatin biology, researchers can uncover new strategies to combat genetic diseases and potentially restore normal cellular function.
Deciphering the Challenges in Gene Expression
While the research on X chromosome inactivation presents exciting therapeutic possibilities, deciphering the challenges in gene expression remains essential. Factors such as gene dosage, epigenetic modifications, and the unique nature of X-linked disorders complicate our understanding of how mutations influence phenotype. For instance, the presence of a healthy gene alongside a mutated copy can create a complex interplay that determines diagnostic outcomes and therapeutic responses.
Moreover, alternate splicing and gene regulation mechanisms introduce layers of complexity that require further investigation. Understanding how these factors interact with X chromosome inactivation can illuminate why certain therapeutic strategies work effectively for some individuals while failing for others. With ongoing research, the aim is to develop comprehensive models that account for these variances, thereby enhancing personalized approaches to treatment.
Linking Basic Research to Therapeutic Applications
The journey from understanding complex biological processes to developing therapeutic applications is a testament to the interconnected nature of modern scientific research. Lee’s long-term commitment to investigating X chromosome behavior exemplifies the need for foundational research in paving the way for new treatment strategies. Central to this effort is the utilization of innovative methods that leverage knowledge about chromosomal interactions to create effective gene therapies.
Connecting basic research in genetics with patient-centric applications amplifies the potential impact on healthcare outcomes for those affected by genetic disorders. As researchers continue to publish their findings and collaborate across disciplines, the culmination of this knowledge forms a robust framework that informs clinical practices. This synergistic relationship between research and therapy signifies a promising path forward in addressing the challenges posed by genetic diseases and ultimately improving the quality of life for affected individuals.
Future Research Directions in Genetics
As the field of genetics continues to evolve, there is an urgent need for future research to address the myriad challenges and questions that remain in understanding gene expression and chromosome behavior. Questions about the extent of Xist RNA’s influence or the mechanisms underlying gene silencing must be prioritized to unravel the complexities of X-linked disorders. Expanding research efforts will provide insights into the broader implications of these findings for various genetic conditions.
Moreover, integrating advances in gene editing technologies, such as CRISPR/Cas9, may offer new avenues to explore targeted therapies that address both gene mutations and expression challenges. By imparting a deeper understanding of genetic regulation and chromosome dynamics, researchers can cultivate innovative approaches that could redefine treatment for genetic diseases. The ultimate goal is to ensure that progress in scientific inquiry translates into tangible benefits for those suffering from genetic disorders.
The Promise of Gene Therapy and Precision Medicine
Gene therapy represents a frontier in modern medicine, with the potential to correct genetic disorders at the molecular level. The insights gained from X chromosome research are particularly significant as they herald the promise of precision medicine—the ability to tailor treatments based on individual genetic profiles. This approach not only enhances the chances of successful interventions but also minimizes adverse effects by focusing on the specific needs of patients.
As we stand on the cusp of this new era in genetic medicine, the integration of findings regarding X chromosome inactivation can enable the development of effective therapies for genetic diseases like Fragile X Syndrome and Rett Syndrome. By advancing research that delves into the complexities of gene expression and leveraging cutting-edge technology, healthcare can become more personalized, addressing the root causes of genetic disorders and fostering better health outcomes for patients worldwide.
Frequently Asked Questions
What is X chromosome inactivation and why is it important for genetic diseases?
X chromosome inactivation (XCI) is a biological process that occurs in females where one of the two copies of the X chromosome is silenced to ensure that gene expression levels are equal between males (who have one X chromosome) and females. This mechanism is crucial for preventing genetic diseases linked to mutations on the X chromosome, such as Fragile X Syndrome and Rett Syndrome. Understanding XCI is vital for developing effective gene therapies aimed at these conditions.
How does the Xist RNA contribute to X chromosome inactivation?
Xist RNA plays a pivotal role in X chromosome inactivation by binding to the X chromosome that needs to be silenced. Once Xist is expressed, it modifies the surrounding chromosomal environment, essentially ‘coating’ the X chromosome in a gelatinous substance that leads to its inactivation. This process facilitates the silencing of genes linked to diseases, including Fragile X Syndrome, offering potential targets for future gene therapies.
Can understanding X chromosome inactivation lead to treatments for genetic diseases like Fragile X Syndrome?
Yes, progressing knowledge about X chromosome inactivation presents promising avenues for treating genetic diseases such as Fragile X Syndrome. Research indicates that by unsilencing active X-linked genes, it may be possible to restore functionality in instances where mutations exist, making XCI a significant target for gene therapy development.
What are the potential implications of freeing inactivated X chromosomes for genetic diseases?
Freeing inactivated X chromosomes could have transformative implications for genetic diseases. By reactivating a healthy gene that is silenced due to X chromosome inactivation, it may be possible to correct the genetic defects seen in conditions like Fragile X Syndrome and Rett Syndrome. This approach could provide a minimally invasive treatment option with reduced side effects as it primarily targets mutated genes.
How are X chromosome inactivation studies aiding research on Rett Syndrome?
Studies on X chromosome inactivation are significantly aiding research on Rett Syndrome by uncovering mechanisms that can potentially unsilence genes responsible for the condition. By manipulating the XCI process, researchers are exploring ways to activate healthy alleles that have been inactivated, which could lead to viable therapeutic strategies for individuals affected by Rett Syndrome.
What makes the X chromosome unique compared to other chromosomes in terms of gene expression?
The X chromosome is unique because females have two copies, necessitating a mechanism like X chromosome inactivation to prevent the overexpression of X-linked genes compared to males, who only possess one copy. This balance is crucial for proper gene dosage and function, making XCI a key focus in the study of genetic diseases linked to the X chromosome.
What is the future of gene therapy related to X chromosome inactivation?
The future of gene therapy related to X chromosome inactivation looks promising, as current research aims to refine techniques that can safely reactivate silenced X-linked genes. Through ongoing studies, there is hope for initiating clinical trials that could lead to new treatments for genetic disorders caused by X-linked mutations, enhancing the quality of life for affected individuals.
| Key Point | Description |
|---|---|
| X Chromosome Inactivation | Females have two X chromosomes, requiring inactivation of one to avoid gene overproduction. |
| Role of Xist RNA | Gene on the X chromosome produces Xist RNA, crucial for modifying the chromosome’s surrounding material, facilitating inactivation. |
| Chromosomal ‘Jell-O’ | A gelatinous substance that both isolates and engages with the X chromosome during inactivation. |
| Therapeutic Potential | Understanding X-inactivation might allow reactivation of healthy genes, providing possible treatments for disorders such as Fragile X and Rett syndromes. |
Summary
X chromosome inactivation is a crucial biological mechanism that regulates gene dosage in females, who possess two X chromosomes compared to males’ one. This intricate process, primarily orchestrated by Xist RNA, modifies the chromosomal environment, leading to the silencing of one X chromosome. Recent advancements in research have uncovered therapeutic potentials, raising hopes for treatments for genetic disorders related to the X chromosome, such as Fragile X and Rett syndromes. Understanding and manipulating X chromosome inactivation could pave the way for innovative therapies and improve lives impacted by genetic conditions.